

Project Momentum
Next Generation Knowledge Economy
Project Momentum
Next Generation Knowledge Economy
Chronicles of
BÖ§ZïK Inc.™ MSE/EPSE
Chronicles of
BÖ§ZïK Inc.™ MSE/EPSE


Project Momentum
Next Generation Knowledge Economy
Project Momentum
Next Generation Knowledge Economy
Chronicles of
BÖ§ZïK Inc.™ MSE/EPSE
Chronicles of
BÖ§ZïK Inc.™ MSE/EPSE
Newsflash! The Schedules for Physics II and
Pure Mathematics I & II are here!
ROOTS of ELECTRICITY
An Elementary Particle
Ad far back as 440 B.C., the atom was was thought of being the “indivisible and indestructible component of an element.” Correcting this notion has prompted the definition of elementary particles, such as electrons, protons, photons, and neutrons.
In essence, an elementary particle is one that cannot be reduced further without losing its physics characteristics. We will focus our initial discussion on one such particle, the electron. The electron is one of the most important of the fundamental, stable and elementary subatomic particles of matter.
The Electronic Charge, e
The most popular model of the atom, as proposed by Ernest Rutherford with supported by the evidence generated in the Geiger-Marsden Gold Foil experiment, is that of a planetary model. The nucleus of the atom, which is dense in mass and positively charged, is surrounded by fast moving negatively charged electrons. Neil Bohr’s work on the atomic model and Max Planck’s contributions have both been phenomenal in the field of Quantum Theory.
Here are some facts about the electron:

Fig. 1. Mysteries of the Atom: driving us to investigate and study the Electronic Charge, e
Modeling Issues
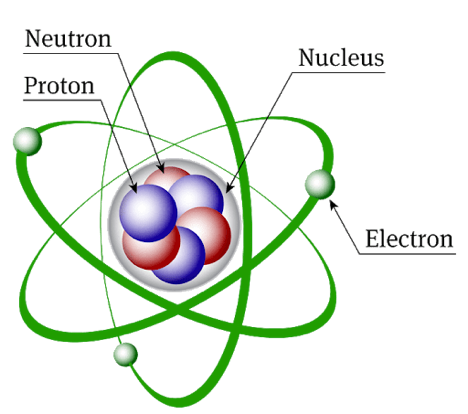
Fig. 2(a). The Planetary Model depicting electrons that orbit a central dense core (the nucleus)
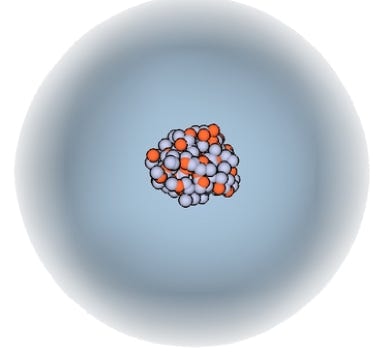
Fig. 2(b). The Cloud Model, where the electronic charges are distributed in a probabilistic space
We will revisit the Atomic Structure later in the course.
Innovation by G. David Boswell
under the auspices of BÖ§ZïK Inc.™ EPSE & MSE Syndicates
Copyright 2020 | All Publication Rights Reserved: Now and Beyond.